There is a course requirement,
That bends the brightest minds.
The mention of its very name,
Sends shivers up young spines!
You might have guessed which course I mean,
It starts with"o" and ends with "ee",
Try not to get too panicky,
It's just organic chemistry!
If you want to learn this well, and easy,
I recommend Baltimore's CCBC,
Dr. DiCara explains things as simple as pie,
(And begins every class with her award winning smile.)
Now here's a rhyme to help you out,
So cast aside your fears and doubts.
I guarantee by time it's done,
You’ll say “Hey! Chemistry is really of fun!"
So here we go one piece at a time,
Please, try to be serious,
(Did I just see you smile?)
And so. Without further delay
Let's begin giving compounds
Their IUPAC names:
The first thing to know about compound names,
Is they’re based on how many carbons
Are In the longest chain.

Ethyl ,methyl, proplyl, butyl,
Learning Greek is never futile.
Pentyl, hexyl heptyl, octyl, non,

Once your carbon count is done
The next step requires counting bonds.
If all the bonds are single,
Then the compound’s an alkane.
Double bonds make alkenes;
And alkine is the triple bond name.
To put these all together now,
Let's name a three carbon chain,
In Greek the three are propyl;
Singly bonded, it's propane.
 |
| propane puppy scultpture |
(With double bonds we'd say its propene,
Triples are propine,
Let's take it for a spin with an eight carbon chain,
Octane, Octene, Octyne.
Now here’s where things start getting fun,
As we progress in this instructional;
Let's talk about a carbon chain,
 |
| Aldehyde vs. Ketone |
Aldehydes and keytones
are a general classification,
That shows how doubly bonded O's
Can change a compounds designation
( depending on the carbonyl carbon's location,
and the double bond's origin and it's destination.)
Like, if the terminal carbon ( on a given chain)
Has a double bonded oxygen, coming off it's side,
And that carbonyl C has a hydrogen, too,
Then the group's called "aldehyde."
And you know how we name aldehydes,
They sound like Pall, Vall and Hall,
So, let's name a three carbon group with an aldehyde ending,
You guessed! It's "propanall."
 |
| Propanal |
And an aldehyde that ends with "H"
Is called an acetal,
Hemi means there's an O-H group,
Without H or O-H it's "ketal."
| Alcohol reacting with Ketone to form Hemiketal and Ketal |

What's a ketal, after all?
It's the group that's derived from ketones,
Ketones have three carbon, all lined up,
And on the carbonyll's a double bonded O.

Well, all this compound naming
Makes you stop, sometimes and blink,
'Cause everything you thought was simple,
Isn't as you think!
Well, if your brain is somewhat straining,
And you'd love to have a drink,
You can fix something real zingy ,
Fizzy, bubbly, spiked, and pink.
Like, say you want to have a drink
That's made with alcohol,
Put O-H on a two carbon chain
And Voila! It's methanol.
 |
| R-O-H Alcohol + a Methyl group= Methyl Alcohol or Methanol |
But if you add an O-H substituant
To a benzene ring,
You've synthesized phenol.
(That's the common name we give to "bezyl alcohol)
 | ||
| Phenol Showing Electrostatic Potential |
Now, alcohol seeks company
In this lonesome big organic world;
If it partners with a double bond O, for drinks,
She gets the reputation of a carboxylic-acid girl.
 |
| Carboxylic Acid |
(But don't forget, it's a two step deal,
Making carboxyls is not so placid;
First C-O-H gets oxidized to doubly bond aldehydes,
Then alde hydrate (hemi-acetals) are oxidized to acid.
 |
| Oxidation of Alcohol ( R-O-H) to Aldehyde ( C=O-H) To Acid (C-O-O-H) |
(And all this, just for a bit of
acid....)
Yet, when it comes to things all sour, carboxylic acids rule the day!
By oxidizing primary alcohols,
C-O-H becomes a doubly bonded acid, hurray.
Now, acids can be oh, so generous,
Despite their bad rep. and sour taste,
They catalyze reaction times,
And donate protons to form conjugate base.
 |
| A Bronsted Acid is a Proton Donor |

 |
| An Arrehnius Acid Dissociates to Form Conjugate Bases and Acids |
 |
| Lea's Cherry Pie |
And here is something quite amazing,
That you might not have guessed, from the start;
An acid can transform into something quite romantic,
Or juicy fruity, or delicious or tart.
You see, if you love the smell of jasmine, yumm,
Banana flavored cherry pie, or rum,
It's cool to know you can synthesize these,
Or ....the flavor of bubble gum!

 |
| Esterfication of a Primary Alcohol |
All you need to do is mix
An acid that's carbolic,
With the right primary alcohol, whichever you wish,
And poof, you've made an Ester, go ahead and whiff.
( 'Cause If you mix carboxylic acid,
With a primary alcohol,
You'll get yourself a new functional group,
Named "Ester", R-C-O-O-R.)
 |
| Dehydration rection of a primary alcohol with one mole of carboxylic acid |
Naming Esters is simple and fun,
You just need to know who's the dad and who's the son;
The acid is the parent, as you know
But COOH loses one bond (uh oh)
that's replaced quickly with the R-group of an alcohol ,and so,
You're left with an "R-carboxyl-oate" (and one mole of H2O.)
 |
| Wintergreen |
Now, if all this talk of compounds,
(What they lose and what they gain,)
Has you clutching your poor forehead
And you feel yourself grow faint,
Just relax we've got you covered,
Smell this oil of wintergreen,
Called methyl salycilate;
Ester of methyl alcohol plus salicylic acid is precisely what you need.
Well, esters are such very cool things,
They' smell like flowers or fruit, or wintergreen,
Saponified carboxylic salt mycels leaves you squeaky ester clean,
And an amide's what you get... when you substitute an amine.
 |
| Dehydration reaction of a carboxy;ic acide with an amine |
Now what's an amine? You might not NO2
'Amine? I don't even kow'ya!',
But this little rascal is your friend from way back when,
If you've ever scrubb-a-dubbed with ammonia.
 |
| Ammonia |
You see, basically, ammonia is
The opposite of a proton donor,It's a Nitrogen atom, with three H's bonded,
(And it also has a 'loner')
This is why it's shape is trigonal pyramidal,
And why it can be ionized a whole lot, or a little.
 |
| Ionization of an amine forms ammonia |
And I know you already know this,
And would never hesitate,
To say that protons in a high dipole state,
 |
| Dipole Moment |
And while we're on the topic
Of proton giving and taking,
Let's talk about Bronstead- Lowry
And the strengths of acids and bases.
An acid is strong when it wants to give it up
To anyone who asks, it's easy,
Remembering these strong acid names
Makes conjugation equations breezy:
Hydrochloric, Hydrobromic,
Nitric, Sufuric, Iodic,
Circle them on your periodic chart,
There isn't much more to it!
Spicy salsa? Five alarm chilly?
What, exactly did you think?
Helapeno` peppers with beer and burritos,
You ate it all but the kitchen sink!



So, If last night you partied hard,
And now your stomach is asking "WHY?",
Before you eat another thing,
Take a swig of antacid Alkline Hydroxide

Sodium, Potassium,
Cesium and Calcium,
Strong bases take all that acids have to give,
While buffers can run back and forth, maintaining pH equilibrium.
And should you ever need to calculate
The concentration of two compounds
When they hit their equilibrium state,
Go ahead, you can do it. ( And this doesn't depend upon rate:)
The equillibrium constant, known as K(eq),
Is something you can calculate, it's not so hard to do;
To know the concentration between reactants and what they make,
Just think about what determines when you give and when you take:
So, to find the concentrations
That reflect the balanced state,
You can talk to old Fritz Haber,
( Don't you wish he were your neighbor?)
He said, to find the point of equillibrium ,
"Tween reactants and what they make,
Equate the concentration of your product
To reactant molarity (times K.)
 |
| Equilibrium constant= molar concentration of product over reactants |
Just remember to raise the coeficients
Into concentration exponents,
And when you report your answer,
Count those sig figs with great dilligence!,
And if you do all this, and keep calm and stay resillient,
I promise you my, orgo friend,
your acuracy will be.....brilliant! "
( Under a high ionic,
constant temperature and pressure
experiemental environment.)
And if you need to know
How fast this happens ( and how slow,)
Divide the change in concentration by how much time has passed,
(For first order reactions,) then plot and calculate the slope.
So now you know the way to show
How much gets made of what,
And how quickly all this happens.
But how can you know the equillibrium point,
When dealing with bases and acids?
Well, Hendersohn and Hasselbeck,
Two guys who were quite classic,
Defined the acid/base equillibrium constant:
As pH plus (the log of) conjugate base over acid.
But enough is enough,
These equations overload my brain!
Instead, let's move onto isomers;
Identical molecular formulas, with different names.
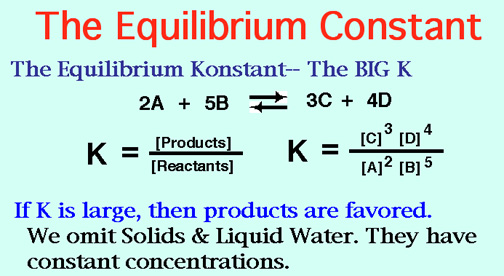
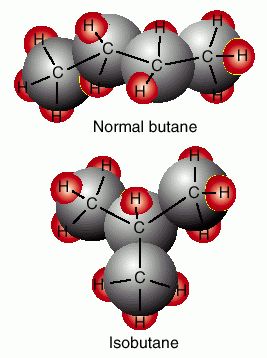
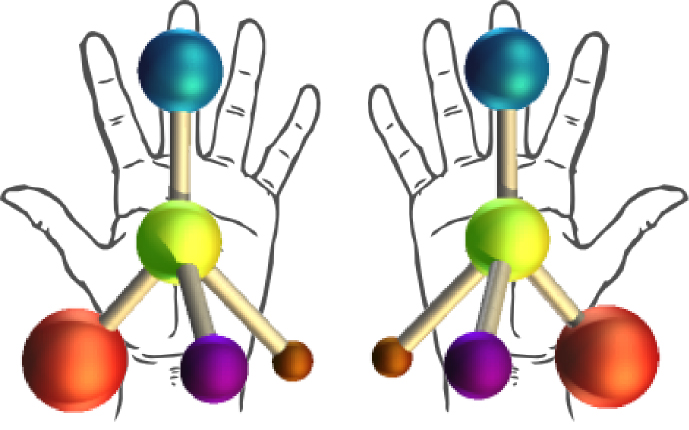
There are three types of isomers
Structural, geometric and chiral
You know that they are isomer's if their formula's are the same
But you'd have to break a bond or two for them to have the same name.

Structural isomers have different bond relationships,
Geometric's are cis and trans,
Chiral molecules are never superimposable,
( Just like your left and right hands.)
  |
| Geometric isomerism Structural Isomerism; Chiral isomerism |
And if you ever need to know,
The number of isomers possible,
What exactly should you do?
I'll answer: just count the chiral carbons at the crossroads, in a row,
Then use that number as you exponent,
Raised up to the base of two.
The number that you find will be,
The value of isomeric possibility.
Well, now that you have had a taste
Of what this course is like,
I hope you fell a bit reassured,
(It's like learning to ride a bike.)
At first it's pretty wobbly,
So many things can go wrong.
But after a while you find yourself riding
So smoothly, while singing this song:
"There is a course requirement,
That bends the brightest minds
The mention of it's very name
Sends shivers up young spines..."
But this time when you sing it,
You will catch yourself with a smile,
Cause now you know, it's not that bad,
If you take it just one carbon at a time!
 |
| H2O |




A large part of Alfa Chemistry's customers are pharmaceutical and biotechnology companies, including Pfizer, Novartis, Merck & Co., Johnson & Johnson, AstraZeneca, and Bayer. Alfa Chemistry is also a preferred partner for many universities and non-profit institutes. TPT1
ReplyDeleteWow, this is amazing!!
ReplyDeleteI like to recommend exclusively fine plus efficient information and facts, hence notice it: how long does it take for cbd gummies to kick in
ReplyDeleteBeaver says I also have such interest, you can read my profile here: How will Vegan CBD Gummies make me feel?
ReplyDelete